Hensin Tsao, MD, PhD
Read BioLaboratory Research Goals
The goal of our laboratory is to understand the molecular basis of melanoma susceptibility, progression and chemosensitivity. Our research platform is anchored in the Wellman Center for Photomedicine, the MGH Melanoma Genetics Program (webpage) and the MGH Melanoma and Pigmented Lesion Center (webpage). Within the Wellman, the Skin Cancer Genetics Laboratory (SCGL) applies state-of-the-art genetic and genomic technologies to elucidate the molecular circuitry driving the melanoma “engine.”

Program in Cancer Genetics and Therapeutics (PCGT)
The Program in Cancer Genetics and Therapeutics (PCGT) is dedicated to uncovering the molecular mechanisms that underlie melanoma predisposition, progression, and therapeutic response. Our mission is to translate fundamental discoveries into tools and strategies that can improve prevention, diagnosis, and treatment of melanoma.
Grounded in a multidisciplinary approach that integrates human genetics, molecular oncology, pharmacology, and bioinformatics, the PCGT seeks to identify individuals at elevated risk, characterize aggressive melanoma subtypes, and develop novel therapies that target the molecular vulnerabilities of the disease.
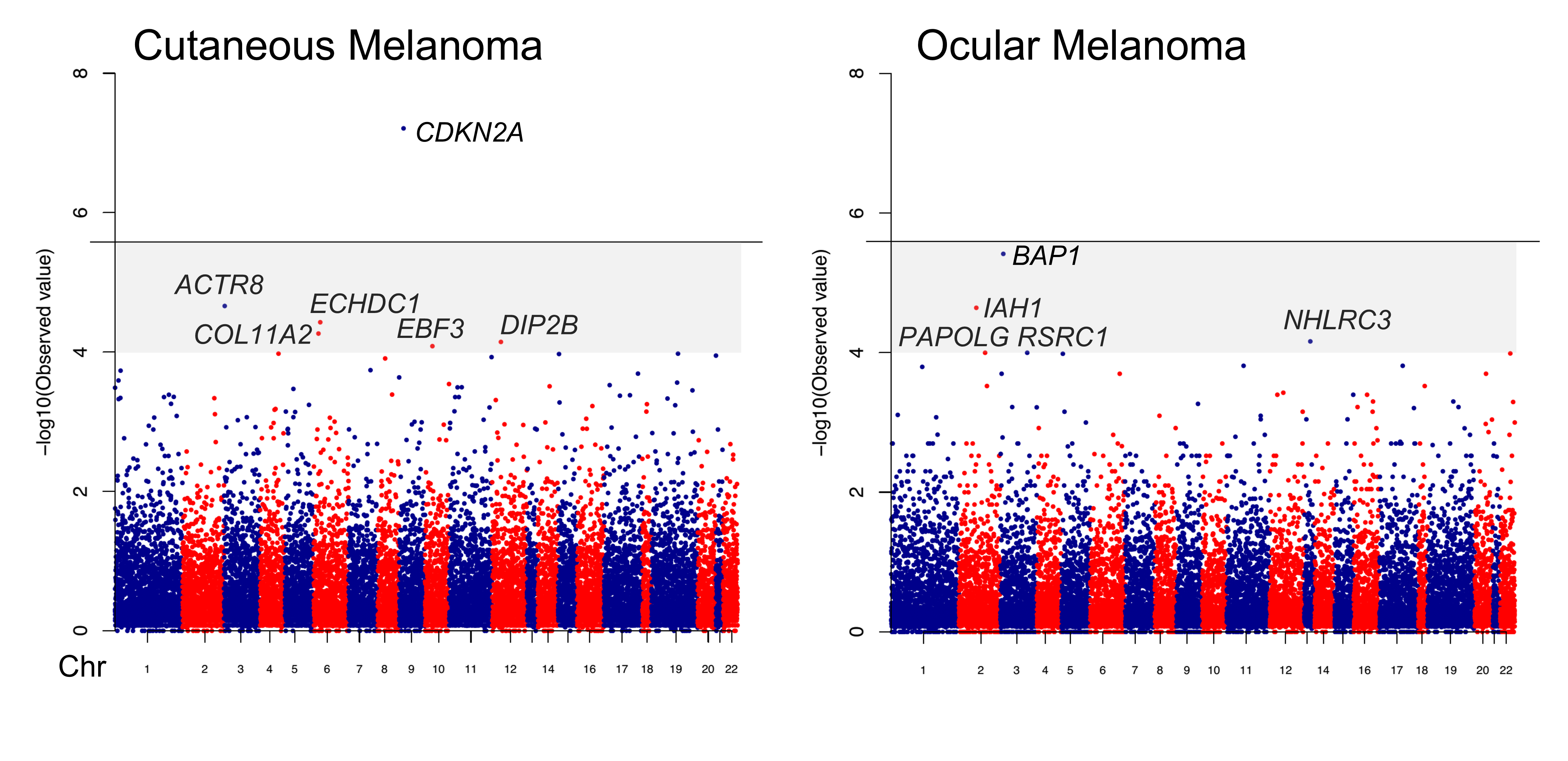
A major emphasis of the PCGT is melanoma susceptibility genetics, where our team has led landmark efforts to identify and stratify individuals based on inherited risk. Our group developed MELPREDICT and MelaPRO, the first clinical decision-support tools designed to estimate the probability of carrying a CDKN2A germline mutation—offering clinicians a quantitative framework for genetic counseling and risk management. Our work also helped describe some of the earliest families harboring germline BAP1 mutations, a discovery that expanded the landscape of familial melanoma syndromes and redefined risk stratification. In collaboration with international partners, we were instrumental in identifying MITF(E318K) as a moderate-penetrance melanoma polymorphism, bridging the gap between high-risk mutations and common variants in melanoma risk. Working with the Broad Institute, we also performed the first gene-based exome-wide association study in melanoma, leading to the identification of EBF3 as a novel melanoma predisposition gene—broadening the genetic framework for assessing melanoma risk.
Our research has also been pivotal in uncovering key molecular drivers of melanoma progression. We were among the first to report PTEN mutations in melanoma and the first to demonstrate functional cooperativity between PTEN loss and activating BRAF mutations in tumorigenesis—a finding that reshaped our understanding of pathway cross-talk in melanoma biology. We also identified EphA2 as an ultraviolet radiation–induced oncogene that not only contributes to melanomagenesis but also mediates resistance to BRAF inhibitors, providing insight into the mechanisms of therapeutic escape in targeted therapy.
The PCGT is also deeply engaged in translational and therapeutic innovation. We developed the first murine melanoma cell line driven by KIT, a gene frequently mutated in acral and mucosal melanoma subtypes. This platform has enabled preclinical modeling of KIT-driven disease and facilitated the creation of a novel hybrid biomimetic nanovaccine, which leverages tumor-derived signals to enhance immune recognition. These efforts exemplify our broader commitment to designing therapeutics that are rooted in the molecular and immunologic context of each melanoma subtype.
Our program’s research has also informed national policy and clinical guidelines. We co-authored the first American Academy of Dermatology (AAD) guidelines on genetic screening for familial melanoma, helping to translate laboratory discoveries into evidence-based recommendations that now guide clinical practice nationwide.
By integrating genomic discovery with therapeutic design and clinical implementation, the PCGT aims to transform the management of melanoma from prevention to cure. Our program remains committed to discovering the biologic and pharmacologic determinants that drive melanoma at every stage—leveraging science to meet the urgent needs of patients and families affected by this aggressive cancer.
Program in Military Health Research (PMHR)
The Program in Military Health Research (PMHR) is focused on advancing our molecular understanding of aberrant wound healing, with a particular emphasis on excessive post-traumatic scar formation and keloids. These conditions represent a critical medical challenge for military personnel, who are often exposed to high-energy injuries, burns, and other trauma in austere and hostile environments. The healing response to such injuries is frequently dysregulated, leading to pathological fibrosis, chronic pain, limited range of motion, and disfigurement—all of which can significantly impair operational readiness, reintegration, and quality of life for service members and veterans. In collaboration with Yonsei University, the PMRH performed one of the largest genome-wide expression analysis of keloids and normal dermal fibroblasts.
The PMHR is also interested in the role of hypoxia in modulating fibrosis and scar formation. Tissue hypoxia is a hallmark of both acute battlefield wounds and the later fibrotic remodeling phase of healing. Under hypoxic conditions, fibroblasts and other stromal cells undergo metabolic and transcriptional reprogramming, largely driven by hypoxia-inducible factors (HIFs), especially HIF-1α.
In response to the military's urgent need for effective countermeasures against pathologic scarring, PMHR is developing and deploying novel phenotypic drug screening platforms that are both physiologically inspired and mechanistically agnostic. While traditional drug discovery approaches often focus on predefined molecular targets, our phenotypic screening models prioritize functional outcomes—such as collagen secretion—within tissue-relevant microenvironments. This approach is particularly valuable for fibrotic diseases, where redundant and overlapping signaling pathways make single-target inhibition often insufficient.
By combining a deep mechanistic understanding of hypoxia-driven fibrotic pathways with cutting-edge translational screening technologies, the PMHR aims to deliver actionable therapies tailored to the unique needs of military personnel. The program envisions a future in which excessive scarring can be predicted, prevented, or reversed—improving the recovery trajectory for injured service members and enabling them to return to duty or civilian life with reduced morbidity and improved function.




Selected Publications
Latoni DI, Kim SH, Tsao H
Systemic Therapies for Metastatic Melanoma. Dermatol Clin. 2025;43(3):483-494 - PMID: 40581428 - DOI: 10.1016/j.det.2025.03.004- More publications ...

